Currently Empty: $0.00
The FDA has released a major update on the agency’s PMTA progress, including publishing the long awaited list of products for which a PMTA application was submitted prior to the September 9th deadline, thus making them legal to sell in the United States–at least for now.
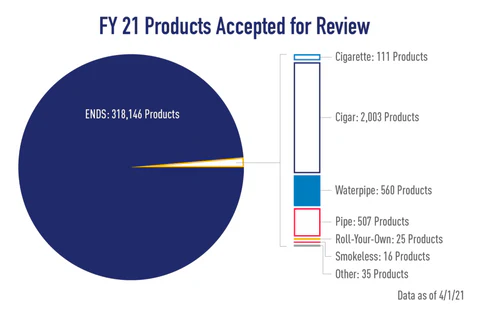
A total of 318,146 vape products have been accepted for review by the FDA to date. That includes all 19 of Kai’s Virgin Vapor’s vape juice flavors submitted!
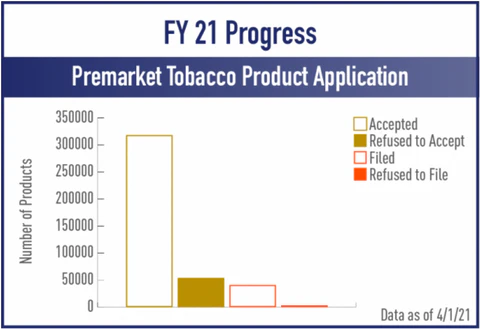
Meanwhile, 54,111 vape products were rejected by the FDA, meaning that the PMTA applications were deemed insufficient by the FDA and the products are no longer allowed on the market. That’s a rejection ratio of 17% which is fairly significant.
Interestingly, no companies have come forward to state that they received a rejection letter from the FDA. However, it’s hard to miss the many vape companies that keep quietly going dark across the net. There’s no way to know if it was the PACT Act, a PMTA rejection letter, or the combined stress and financial pressures of COVID and other factors present over the past year that caused the shut down.
The next hurdle for companies lucky enough to be on FDA’s acceptance list is filing. The FDA is reviewing PMTA’s for the 318,146 products that have advanced through round one, taking a closer look at the information presented to see if it is sufficient to file the application and allow it to advance to the scientific review phase.
A fair number of products, 41,056 to be exact, a list occupied generally by companies who filed further in advance of the September 9th deadline, have already received filing letters and are making their way through scientific review. But 3,488 products that were accepted for review did receive refuse to file letters, ending the PMTA hopes and dreams of their respective companies.
Of the companies currently in scientific review, 366 of them have already received deficiency letters from the FDA. While the name “deficiency letter” sounds ominous, receiving a deficiency letter from the FDA is to be expected. In fact, even PMTA’s put together by Big Tobacco, and written by virtual armies of scientists and lawyers, receive deficiencies. Receiving and addressing the deficiencies found is an expected part of the process for all vape companies.
Despite the progress, the number of vape products that have received a marketing order from the FDA is still zero but we hope to see that number change as products work their way through FDA review.
Please keep Kai’s Virgin Vapor in your thoughts and prayers!
